is hydrogen chloride polar Polar hydrogen chloride hcl bonds
Hydrogen chloride and hydrochloric acid are two terms that often get used interchangeably in everyday conversation. However, there is a difference between the two. Let’s explore the dissimilarities and understand the underlying concepts. To begin with, hydrogen chloride (HCl) is a chemical compound made up of hydrogen and chlorine. It is a colorless gas that is highly soluble in water. On the other hand, hydrochloric acid (HCl) refers to the aqueous solution of hydrogen chloride gas. When hydrogen chloride gas dissolves in water, it forms hydrochloric acid. The main distinction between hydrogen chloride and hydrochloric acid lies in their physical states. While hydrogen chloride is a gas at room temperature, hydrochloric acid exists as a liquid. The presence of water molecules in hydrochloric acid allows it to be in a liquid form rather than a gas. In terms of chemical properties, both hydrogen chloride and hydrochloric acid are highly acidic substances. However, due to the presence of water, hydrochloric acid is more corrosive and potent than hydrogen chloride gas. This increased reactivity of hydrochloric acid makes it a versatile compound in various industrial and laboratory applications. Now, let’s delve into the polarity of hydrogen chloride. Polarity refers to the distribution of electrical charge within a molecule. It plays a crucial role in determining the physical and chemical behavior of a compound. In the case of hydrogen chloride, the molecule is polar. The polarity of hydrogen chloride arises from the difference in electronegativity between hydrogen and chlorine atoms. Chlorine is significantly more electronegative than hydrogen, meaning it has a stronger pull on the shared electrons. As a result, the chlorine atom acquires a partial negative charge (δ-) while the hydrogen atom carries a partial positive charge (δ+). This polarity allows hydrogen chloride to form strong intermolecular forces, such as hydrogen bonding, which affects its physical properties such as boiling and melting points. Additionally, the polar nature of hydrogen chloride enables it to dissolve in polar solvents like water. To visualize the structure of hydrogen chloride, let’s take a look at the image below.  As you can see, the hydrogen chloride molecule consists of a hydrogen atom bonded to a chlorine atom. The electronegativity difference between the two atoms leads to the formation of a polar covalent bond. In summary, hydrogen chloride and hydrochloric acid are related but distinct terms. Hydrogen chloride refers to the gas phase compound, while hydrochloric acid denotes the solution of hydrogen chloride in water. Hydrogen chloride is a polar molecule, and its polarity influences its physical and chemical properties. Understanding these differences between hydrogen chloride and hydrochloric acid is essential in various scientific and industrial fields.
If you are looking for Is Hydrogen Chloride (HCl) Polar or Nonpolar? (Why? & How?) you’ve visit to the right place. We have 5 Pictures about Is Hydrogen Chloride (HCl) Polar or Nonpolar? (Why? & How?) like Polar bond in hydrogen chloride molecule - Stock Image - C028/6490, Is Hydrogen Chloride (HCl) Polar or Nonpolar? (Why? & How?) and also Polar bond in hydrogen chloride molecule - Stock Image - C028/6490. Here you go:
Is Hydrogen Chloride (HCl) Polar Or Nonpolar? (Why? & How?)
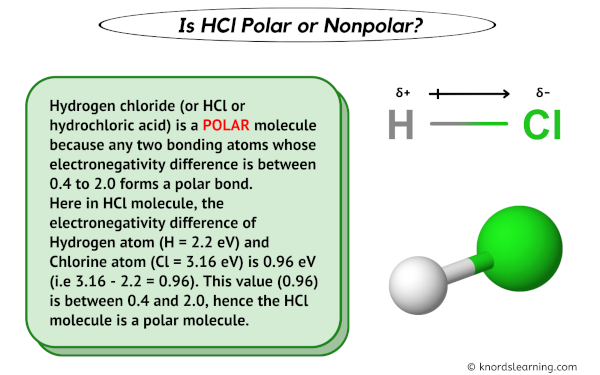 knordslearning.comPolar Bond In Hydrogen Chloride Molecule - Stock Image - C028/6490
knordslearning.comPolar Bond In Hydrogen Chloride Molecule - Stock Image - C028/6490
 www.sciencephoto.comhydrogen chloride molecule libary
www.sciencephoto.comhydrogen chloride molecule libary
Polar Bond In Hydrogen Chloride Molecule Photograph By Animate4.com
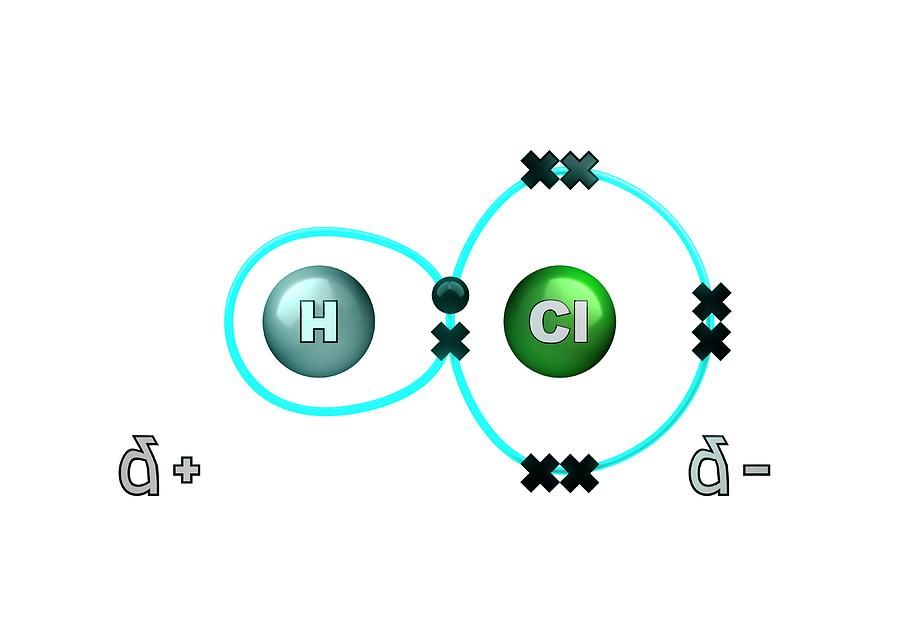 pixels.comchloride hydrogen molecule polar bond libary science photograph illustration uploaded june which
pixels.comchloride hydrogen molecule polar bond libary science photograph illustration uploaded june which
Difference Between Hydrogen Chloride And Hydrochloric Acid | Definition
 pediaa.comhydrogen chloride acid binary hydrochloric acids oxyacids hcl between difference polar molecule pediaa distinguish figure chemical
pediaa.comhydrogen chloride acid binary hydrochloric acids oxyacids hcl between difference polar molecule pediaa distinguish figure chemical
Chapter 3
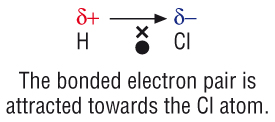 www.chemhume.co.ukpolar hydrogen chloride hcl bonds
Hydrogen chloride acid binary hydrochloric acids oxyacids hcl between difference polar molecule pediaa distinguish figure chemical. Chloride hydrogen molecule polar bond libary science photograph illustration uploaded june which. Is hydrogen chloride (hcl) polar or nonpolar? (why? & how?)